Constipation affects up to 15% of adults, yet distinguishing between pelvic-floor outlet obstruction and slow-transit dysfunction remains challenging without objective testing. Balloon expulsion test (BET) is a cornerstone diagnostic method—but only if the balloon is calibrated, patient-safe, and easy to use within standard motility workflows.
MUI Scientific Anorectal Balloon Catheters, available from Minerva Health Solutions, meet these demands with calibrated volume markers, non-DEHP materials, and EO-sterile single-use packaging. This comprehensive review covers clinical evidence, product features, and practical workflow integration—helping clinicians drive reliable data, faster diagnoses, and better patient outcomes.
Why Standardized Balloon Expulsion Matters
1. Evidence-Based Diagnosis
-
Rome IV guidelines recommend BET for confirming outlet obstruction in chronic constipation.
-
Standardizing balloon size and inflation volume reduces false negatives and enhances diagnostic confidence.
2. Reproducibility & Comparability
-
Multi-center studies reveal that balloon material, fill media, and volume differences introduce up to 25% variability in expulsion time.
-
Using the same catheter type across clinics enables consistent data for clinical trials and quality-improvement registries.
3. Infection Prevention
-
Outbreak reports link reusable anorectal catheters to cross-contamination.
-
Single-patient, EO-sterile devices like MUI Scientific mitigate this risk, protecting patients and staff while addressing Joint Commission audits.
Meet the Anorectal Balloon Catheter Line
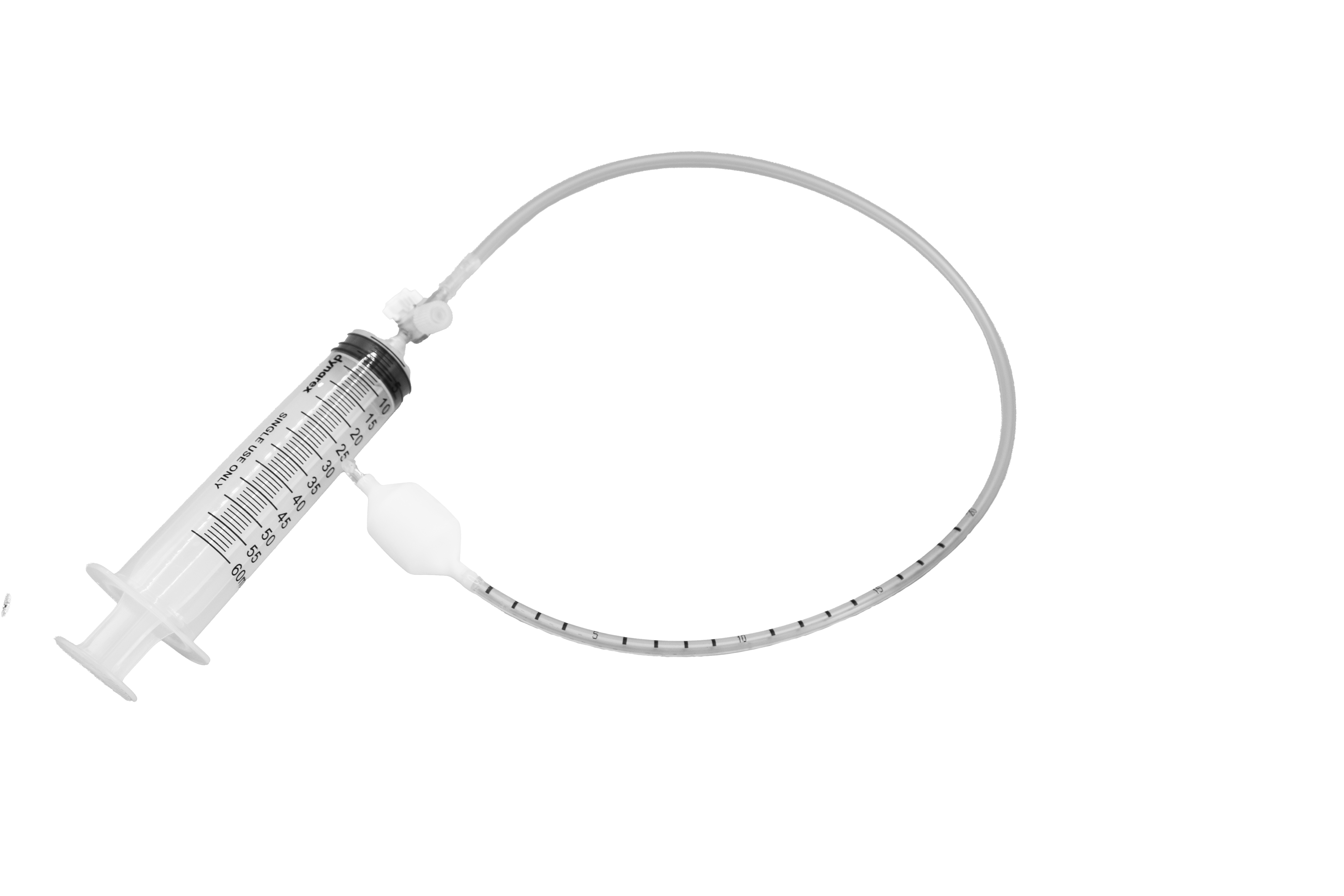
| Feature | SR1B | SR2B | Clinical Benefit |
|---|---|---|---|
| Balloon Material | Medical-grade PVC, non-DEHP | Medical-grade PVC, non-DEHP | Maintains compliance curve for accurate sensory thresholds |
| Inflation Media | Air only | Air or water | Flexible protocol options |
| Balloon Capacity | 400 mL max; 30 mL increments printed | 400 mL max; same increments | Prevents over-inflation & mucosal trauma |
| Tubing Length | 60 cm (OD 5.7 mm) | 110 cm | Connects to HRM systems without signal noise |
| Sterility | EO-sterile, single-use only | EO-sterile, single-use | Eliminates re-processing delays |
-
Compare SR1B and SR2B in detail: SR2B product page
Key Design Advantages
-
Non-latex chloroprene balloon—suitable for latex-sensitive patients.
-
High-contrast volume markers—enable quick, accurate inflation.
-
Universal Luer connector—fits all popular HRM systems for seamless integration.
Clinical Evidence & Regulatory Compliance
Peer-Reviewed Data
-
2024 study (n=60): PVC balloons produced sensory thresholds within 5% of latex controls, eliminating allergen risk.
-
Meta-analysis (five trials, n=312): Calibrated BET reduced misclassification of dyssynergic defecation by 17% compared to uncalibrated balloons.
Regulatory Status
-
FDA 510(k)-cleared as accessories for anorectal manometry and BET.
-
Conform to ISO 10993 biocompatibility and ANSI/AAMI ST72 sterility validation.
-
Non-DEHP construction meets EU MDR and U.S. hospital mandates on phthalate reduction.
Quality Assurance
-
Every batch tested for burst pressure and dimensions.
-
Laser-etched lot numbers enable full traceability for CAP-accredited motility labs.
Integrating MUI Catheters into Your Workflow
Step-by-Step Protocol
-
Preparation
-
Verify packaging integrity and expiration date.
-
Assemble 3-way stopcock and 60 mL syringe (included).
-
-
Patient Positioning
-
Left lateral decubitus; knees flexed to aid anal access and sphincter relaxation.
-
-
Catheter Insertion
-
Lubricate tip; advance 8–10 cm proximal to the anal verge.
-
-
Balloon Inflation
-
Inject 50 mL air (standardized) using marked syringe.
-
Record time to expulsion; if >2 min, allow patient to sit on commode for completion.
-
-
Post-Procedure
-
Document time and discomfort; dispose used catheter—no re-sterilization.
-
Integration Tips
-
Bundle with high-resolution manometry (HRM) for one-visit motility work-ups.
-
Stock both SR1B and SR2B to support air-only and water-perfused protocols.
Frequently Asked Questions (FAQ)
Q1. Do these catheters work with my HRM console?
Yes. The female Luer-lock connector fits standard solid-state and water-perfused ports. 60–110 cm tubing helps prevent signal artifacts.
Q2. Are MUI balloons suitable for pediatric use?
SR1B’s slim 5.7 mm OD and controlled inflation make it suitable for adolescent BET; for younger children, consult Minerva’s pediatric motility catheter line.
Q3. Can I re-process the catheter to save costs?
No. The product is validated for single-patient use only. Re-processing compromises elasticity and violates FDA labeling.
Q4. How do I choose between SR1B and SR2B?
Select SR1B for routine air inflation; SR2B supports water-perfused studies and rectal compliance testing.
Q5. Does non-DEHP material affect balloon compliance?
Bench testing confirms non-DEHP PVC maintains identical compliance curves to conventional DEHP PVC—up to 400 mL.
Testimonials & Case Insights
“Switching to MUI’s non-DEHP catheter cut our test-setup time by 30% and helped us pass a surprise infection-control audit.”
— Dr. Kim, MD
“The clearly printed volume markers virtually eliminated operator error in our motility lab.”
— Susan, RN, Motility Coordinator
These results show efficiency gains and regulatory peace of mind—benefits your institution can realize immediately by standardizing MUI Scientific products.
Robust GI motility data begins with the right tools. MUI Scientific Anorectal Balloon Catheters, through Minerva Health Solutions, deliver calibrated volumes, non-DEHP patient safety, and FDA-cleared performance. Empower your team to pinpoint outlet obstruction accurately, streamline workflows, and elevate patient care.
-
Request a Demo: Minerva Contact Form
-
Get SR1B & SR2B Spec Sheet (PDF) - sales@minervahealthinc.com
-
Schedule a Virtual In-Service for your team
Disclaimer
This blog is for informational purposes only and does not substitute for professional medical advice. Always follow official Instructions for Use (IFU) and institutional protocols.
Got questions? Want to learn more? Get in touch with us today! We’re here to support you with top-quality medical equipment and supplies that help you provide the best patient care possible - sales@minervahealthinc.com or +1 (833) 464-6378.