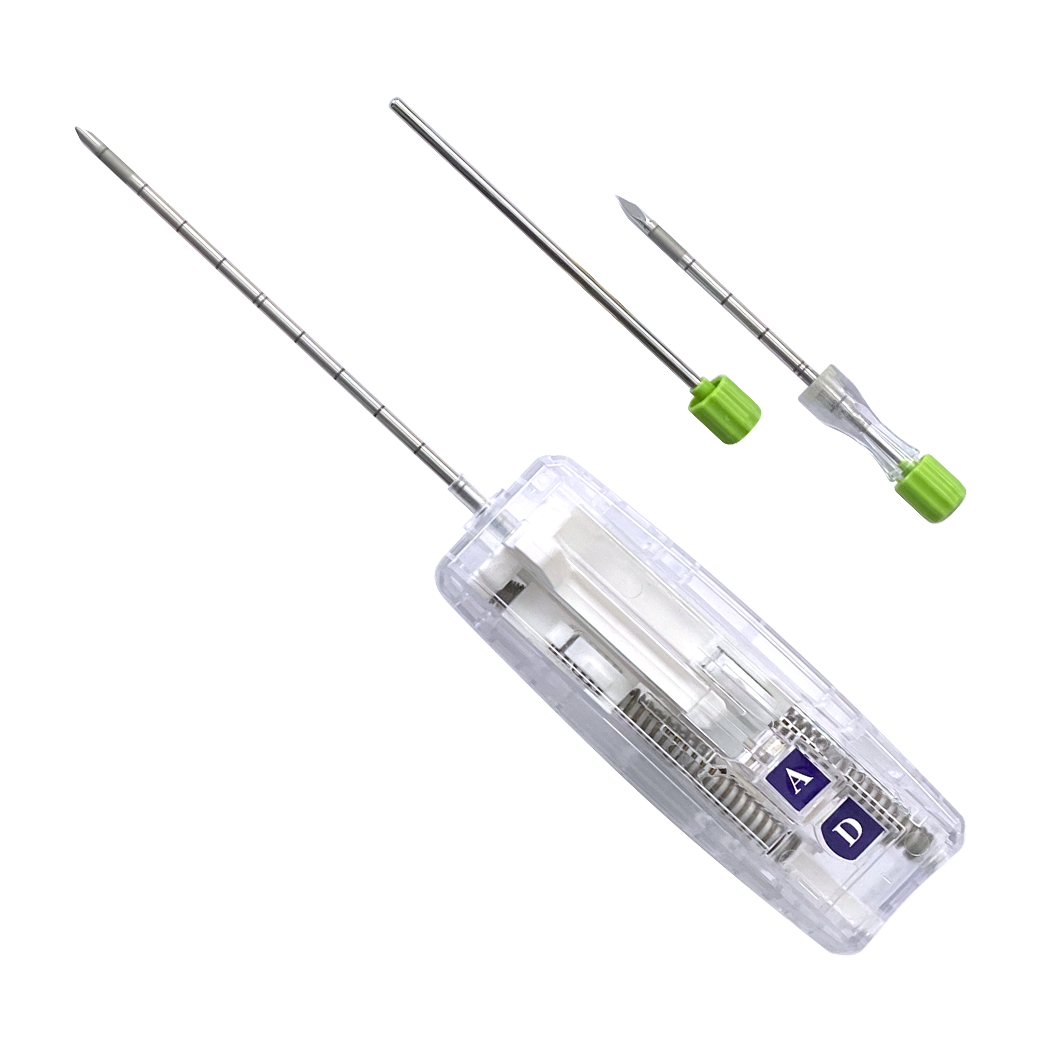
Recent breakthroughs in breast lesion localization and biopsy are reshaping how clinicians approach diagnosis, minimally invasive sampling, and precision surgery. With breast cancer detection relying more on screening and imaging, nonpalpable lesions have become a significant challenge for radiologists, surgeons, and pathologists. Minerva Health Solutions delivers state-of-the-art biopsy needles and localization systems designed for efficiency, accuracy, and compliance in modern clinical practice.
Product Portfolio Overview
Minerva Health Solutions offers a robust biopsy and breast lesion localization range, including:
-
Quick-Core Auto Coaxial Biopsy Needle Set
-
Quick-Core Biopsy Needles (auto and manual)
-
Quick-Core Coaxial Biopsy Needle Set
-
X-Reidy Breast Lesion Localization Needle
-
Jabczenski Ductogram Cannula
-
Muller-Schimpfle MMS Echotip Breast Localization Coil
-
Kopans Modified Breast Lesion Localization Needle
Explore the full product collection at Minerva Health Solutions.
Key Features & Clinical Technologies
Quick-Core Biopsy Needles
-
Device Options: Semi-automatic and fully automatic models for sample precision and workflow optimization.
-
Needle Selection: Multiple gauges (12G–20G), coaxial design, customizable lengths fitting various anatomy and imaging protocols.
-
Programmable Firing: Fully automatic device with three configurable settings to match speed and sample size needs.
-
Compatibility: Optimized for ultrasound, mammography, and MRI guidance.
Breast Lesion Localization Wires & Needles
-
Kopans Modified Localization Needles: Widely used for pre-surgical wire localization; adaptable for deep and superficial lesions.
-
X-Reidy Localization Needles: High-precision marking with radioopaque features for imaging visibility.
-
Muller-Schimpfle MMS Echotip Coil: Specialized for improved ultrasound visibility and anchor stability.
-
ColdCare Packs: Enhance comfort and healing post-biopsy or localization.
Evidence-Based Comparison & Clinical Trends
Wire localization remains the gold standard for nonpalpable breast lesion management, especially when surgical excision is required. Devices like Kopans and X-Reidy needles dominate the market due to proven accuracy, cost-effectiveness, and procedural familiarity. Recent market insights show wire localization with over 30% North American market share in 2025.
However, innovation is rapid. Magnetic seed tracers, ultrasound-visible coils, and MRI-compatible devices offer enhanced workflow and patient-centricity. Clinician surveys indicate growing preference for minimally invasive, image-guided strategies such as:
-
Core Needle Biopsy (CNB): High diagnostic yield for solid lesions (accuracy 79–99% for malignancy).
-
Vacuum-Assisted Breast Biopsy (VABB): Ensures comprehensive sampling, 360° tissue retrieval, and lower re-biopsy rates.
-
Digital Breast Tomosynthesis (DBT) and MRI Guidance: Enable accurate targeting of small, deep, or complex lesions.
Minerva Health Solutions incorporates these trends by offering versatile, regulatory compliant products suitable for a wide spectrum of clinical environments and patient needs.
Frequently Asked Questions (FAQ)
Q1: Which breast lesions require localization?
A1: Nonpalpable lesions identified through screening ultrasound, mammography, or MRI typically require wire or coil localization prior to biopsy or surgical excision.
Q2: What Minerva product is best for ultrasound-guided procedures?
A2: The Muller-Schimpfle MMS Echotip Coil and Quick-Core Auto Biopsy Needles are ideal, both offering enhanced ultrasound visibility and customizable gauge options.
Q3: Are Minerva’s biopsy and localization devices FDA-approved?
A3: All listed products comply with FDA and CE regulatory standards; documentation available on request.
Q4: How does wire localization compare to seed or magnetic localization?
A4: Wire localization is cost-effective, well studied, and widely adopted. Magnetic and seed-based methods reduce radiation exposure and can improve patient comfort but require additional training and resource investment.
Q5: Can clinicians request a demo or clinical trial kit?
A5: Yes. Complete the contact form on the product page or request a clinical sample directly from Minerva Health Solutions.
Clinical Benefits for Breast Care Teams
-
Improved Diagnostic Accuracy: Programmable needle firing, precision marking, and ultrasound/MRI compatibility reduce false negatives and sampling errors.
-
Patient-Centric Design: Ergonomic, minimally invasive biopsy systems; cooling care products for enhanced post-procedure comfort.
-
Reduced Procedure Time: Streamlined devices for one-step wire deployment and easy tissue capture lower operative times.
-
Regulatory Confidence: All devices manufactured and documented to meet safety, tracking, and compliance standards in North America and the EU.
Case Studies & Physician Testimonials
“The Quick-Core device improved our lesion sampling accuracy and workflow efficiency in outpatient procedures.”
— Dr. Rao, Interventional Radiologist
“Breast localization wires from Minerva have streamlined our surgical planning for nonpalpable lesions, improving patient outcomes and staff confidence.”
— Dr. Chen, Breast Surgeon
Minerva Health Solutions welcomes additional physician feedback and collaboration for ongoing product innovation.
-
Request a demo of Minerva biopsy and localization products.
-
Contact sales for clinical solutions tailored to practice needs.
-
Learn more about Minerva’s breast care portfolio today.
Got questions? Want to learn more? Get in touch with us today! We’re here to support you with top-quality medical equipment and supplies that help you provide the best patient care possible - sales@minervahealthinc.com or +1 (833) 464-6378.