Introduction: Where urethral bulking fits in SUI care
Urethral bulking has re‑emerged as a meaningful option for women with stress urinary incontinence who have failed conservative therapy but are poor candidates for, or reluctant to undergo, sling surgery. Contemporary PubMed‑indexed reviews and long‑term cohort data show that modern bulking agents, particularly polyacrylamide hydrogel (PAHG, Bulkamid‑type), can deliver durable symptom improvement in a substantial proportion of carefully selected patients, including those with recurrent SUI after prior procedures.
For clinicians, the key is not whether bulking “replaces” mid‑urethral slings, but how to position it within an individualized care pathway that respects patient preference, comorbidity burden, and prior surgery history. The sections below synthesize current evidence to support patient selection, counselling, and follow‑up.
Minerva Health Solutions offers the Bulkamid‑style Cystoscope 2.7 mm x 113 mm, 0‑degree (41‑0152A), engineered with Tru‑Vivid HD optics and Bulkamid‑compatible design to deliver high‑definition visualization during urethral bulking procedures.
Mechanism, agents, and technique
How urethral bulking works
- Urethral bulking agents (UBAs) are injected in the proximal or mid‑urethra to increase submucosal volume, improving mucosal coaptation and urethral closing pressure during rises in intra‑abdominal pressure.
- This coaptation reduces leakage under stress without significantly altering detrusor function, making UBAs particularly suited to stress‑predominant incontinence rather than pure urgency incontinence.
Contemporary agents
Recent clinical reviews and trials highlight several categories of UBAs:
- Polyacrylamide hydrogel (PAHG, Bulkamid)
- Non‑particulate, hydrophilic, non‑biodegradable gel comprised of cross‑linked polyacrylamide and water.
- Integrates with host tissue through fine fibrous ingrowth, with minimal migration and low inflammatory response in long‑term series.
- Particulate agents in a carrier gel (e.g., polydimethylsiloxane such as Macroplastique)
- Provide structural bulk via solid particles; historical concerns about migration and granuloma formation have been mitigated but not entirely eliminated.
Reviews from 2024 emphasize PAHG as the most extensively studied modern agent with robust 7‑year outcome data and a favorable safety profile.
Procedural technique (high‑level)
- Most series describe endoscopic injection via a rigid or flexible cystoscope, using a dedicated injection needle at the proximal third of the urethra, typically at 3–4 quadrants.
- Local anesthesia with or without sedation is common, with the procedure performed in an outpatient or ambulatory setting; patients are usually discharged the same day after at least one observed void.
Indications, patient selection, and special scenarios
Core indications
A 2024 clinical practice statement and multiple systematic reviews converge on similar indications:
- Women with demonstrable stress urinary incontinence (or stress‑predominant mixed incontinence) in whom conservative measures such as pelvic floor muscle training have failed.
- Patients who desire a minimally invasive option, accept the likelihood of repeat injections, and either are not ideal candidates for mid‑urethral sling or strongly prefer to avoid mesh surgery.
Intrinsic sphincter deficiency (ISD) may coexist but should not be the sole indication; the overall clinical picture and patient goals should drive decision‑making.
Salvage after failed continence surgery
- A 2025 review of UBAs as salvage therapy for recurrent SUI (rSUI) after prior sling or colposuspension found that bulking can deliver meaningful symptom improvement with less morbidity than repeat sling or autologous fascial procedures.
- In these cohorts, cure rates are lower than in primary SUI, but a substantial fraction of patients report clinically important reductions in pad use and bother, making UBAs a pragmatic option when additional major surgery is undesirable.
Neurologic and complex patients
- A recent trial in women with multiple sclerosis reported that combining urethral bulking with pelvic floor muscle training improved SUI symptoms and quality‑of‑life scores, with acceptable safety.
- These findings support the use of bulking in selected neurologically complex patients where more invasive reconstructive procedures carry increased risk or lower acceptability.
Efficacy, durability, and comparative outcomes
Long‑term data for PAHG (Bulkamid‑type)
- The 7‑year Bulkamid study (388 women with long‑term follow‑up) reported that 67.1% of patients treated as a primary procedure described themselves as “cured or improved,” with significant and sustained reductions in ICIQ‑UI SF scores.
- Complication rates were low, with no reports of material migration or systemic reactions, and most adverse events were mild and transient (e.g., dysuria, urinary tract infection, transient retention).
Broader effectiveness across agents
- A 2024 narrative review (“Current Treatment of Stress Urinary Incontinence by Bulking Agents”) concluded that UBAs achieve clinically relevant improvement in roughly 50–70% of women, with higher subjective satisfaction in carefully selected SUI or stress‑predominant mixed incontinence patients.
- A separate evidence synthesis on bulking agents confirmed that, while objective cure rates are generally lower than those of mid‑urethral slings, patient‑reported improvement and satisfaction can be high when expectations are appropriately managed.
Mixed incontinence and OAB symptoms
- A 2024 study of Macroplastique in women with mixed urinary incontinence reported that a notable proportion experienced improvement in urgency and urgency incontinence, with only about 15% requiring additional overactive bladder therapy at one year.
- The authors propose that improved bladder‑neck support and reduced funneling may modulate afferent signaling, partially explaining the effect on urgency symptoms in some patients.
Risks, complications, and follow‑up considerations
Common adverse events
Across PubMed‑indexed cohorts and trials, the safety profile of modern UBAs is generally favorable:
- Transient dysuria, frequency, and microscopic or mild macroscopic hematuria are frequent but short‑lived.
- Urinary tract infection and short‑term urinary retention occur in a minority; most retention cases resolve with brief catheterization or observation.
Serious events such as erosion, abscess, or distant migration are rare with PAHG but have been historically reported with some particulate agents, highlighting the need to understand the specific product profile being used.
Re‑bulking and subsequent surgery
- Re‑bulking is common; in long‑term Bulkamid series, many women received more than one injection over the follow‑up period, typically triggered by persistent or recurrent SUI.
- Some patients ultimately proceed to sling surgery; importantly, current evidence suggests that prior PAHG injection does not preclude subsequent mid‑urethral sling placement, though careful surgical planning is advised.
Counselling points for clinicians
- Frame urethral bulking as a minimally invasive, repeatable option with a strong safety profile but a lower “one‑and‑done” cure probability than slings.
- Emphasize that improvement—rather than complete dryness—is a realistic and common outcome, and that repeat injections or later surgery remain on the table if symptoms recur.
Practical takeaways for clinical practice
- Reserve urethral bulking primarily for women with demonstrable SUI or stress‑predominant mixed incontinence who value minimal invasiveness, rapid recovery, and are comfortable with the trade‑off of potentially lower long‑term cure rates.
- Prefer well‑studied modern agents such as PAHG (Bulkamid‑type), which offer robust long‑term safety and effectiveness data, especially in primary SUI and salvage settings.
- Integrate structured follow‑up with objective measures (e.g., pad tests, validated questionnaires) and clear criteria for re‑bulking or escalation to sling, aligning expectations with the evidence base summarized in contemporary PubMed‑indexed literature.
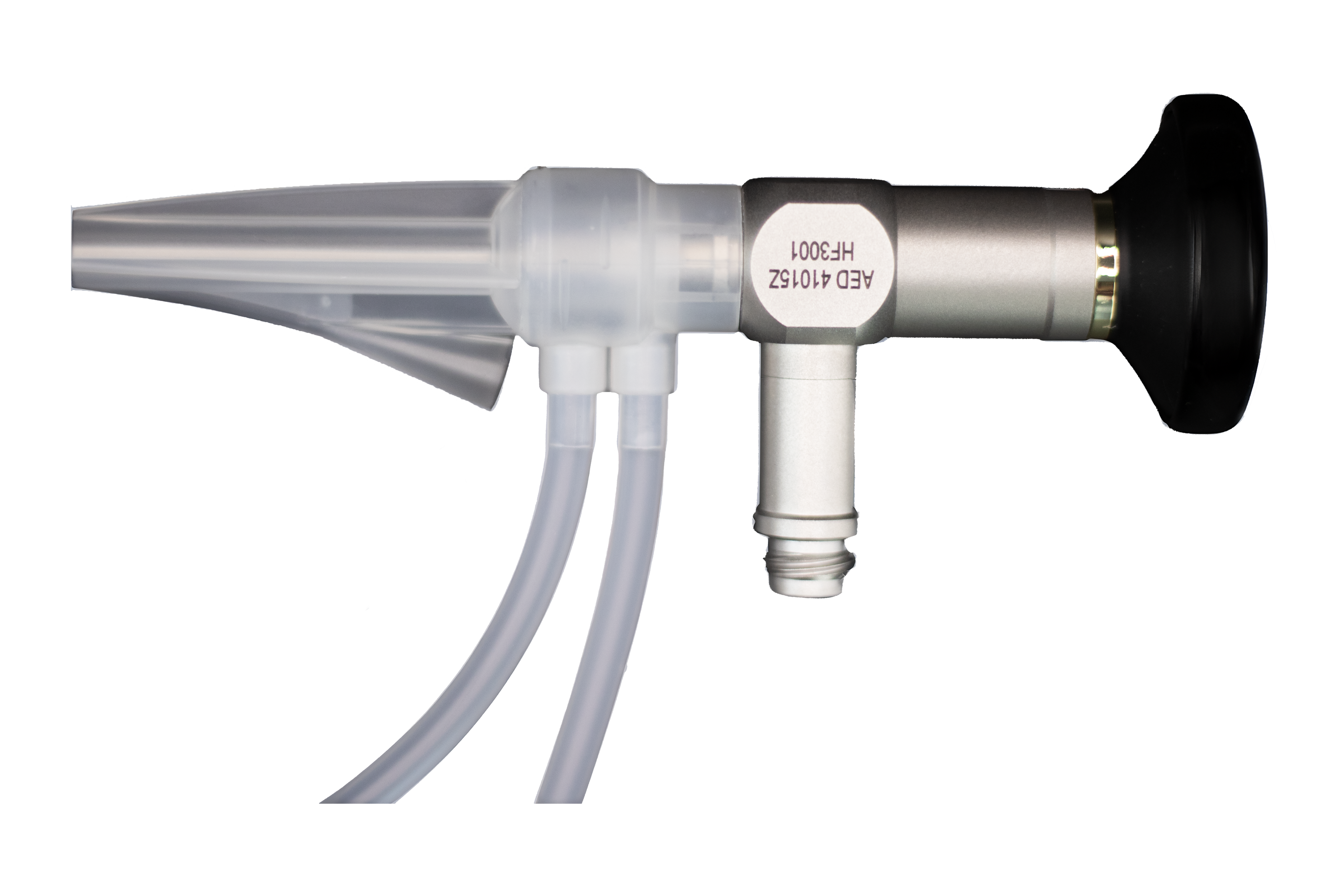
Bulkamid‑style 2.7 mm, 0‑degree cystoscope
To support precise urethral bulking, Minerva Health Solutions offers a Bulkamid‑style cystoscope specifically designed for these procedures: the 2.7 mm x 113 mm, 0‑degree model 41‑0152A.
Key technical features relevant to bulking workflows include:
- Slim diameter (2.7 mm) – Minimally invasive access suitable for urethral bulking and other small‑caliber urologic procedures.
- Working length 113 mm and 0‑degree direct view – Provides a straight, forward visualization of the urethral lumen and bladder neck ideal for locating the proximal urethra and guiding injections.
- Tru‑Vivid HD rod lens system – High‑definition, distortion‑free imaging to clearly visualize mucosa, injection blebs, and final coaptation.
- Soldered sapphire distal lens and high‑aperture light fibers – Scratch‑resistant optics and strong illumination to maintain image quality over repeated sterilization cycles and procedures.
- Compatibility with the Bulkamid sheath (10087‑001) and standard light‑cable adapters – Facilitates integration into existing bulking setups and supports manufacturers’ standard operating procedures for Bulkamid urethral bulking systems.
Clinically, this configuration supports accurate, reproducible injection technique, which is critical for achieving the coaptation and outcomes reported in long‑term Bulkamid cohorts.
This blog is for informational purposes only and does not replace individualized clinical judgement or guideline‑based decision‑making in the management of urinary incontinence.